Recently, the matters stipulated in the Act on Securing the Quality, Efficacy and Safety of Pharmaceuticals and Medical Devices (hereinafter referred to as the “Pharmaceuticals and Medical Devices Act”) have been implemented for cosmetics imported into Japan from overseas in the city. Cosmetics are regulated by the Pharmaceuticals and Medical Devices Law, and the items stipulated by the Pharmaceuticals and Medical Devices Law (name of ingredient, name of cosmetics manufacturer and address, manufacturing number, etc.) must be written in Japanese .
The act of selling cosmetics that do not meet the statutory labeling requirements , such as the lack of Japanese descriptions, is a violation of the Pharmaceuticals and Medical Devices Act. (Violation of Article 55, Paragraph 1 of the Act)
In addition, it is necessary to obtain a license for the cosmetics manufacturing business or the cosmetics manufacturing and sales business in order to ship products imported from overseas as cosmetics to the domestic market .
Labeling of cosmetics (example)
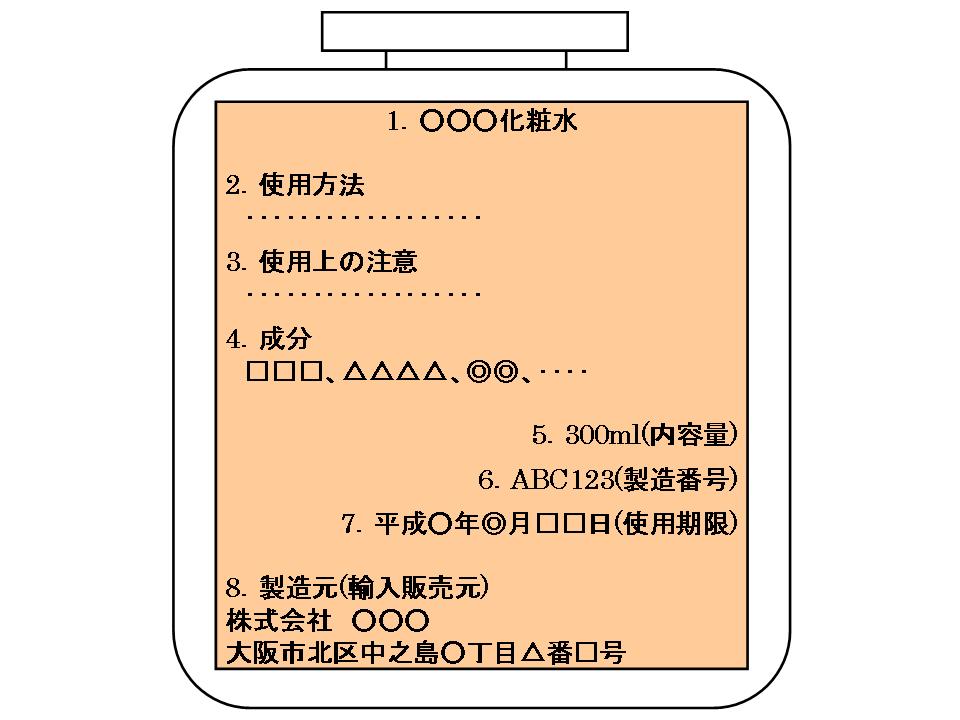
Items to be described on the direct container, etc.
(Articles 52 and 61 applied mutatis mutandis to Article 62 of the Pharmaceuticals and Medical Devices Law)
1. Product name
2. Dosage
3. Precautions necessary for handling
Four. All component names
Five. Contents such as weight, capacity or number*
6. Production number or production code
7. Expiration date※
8. Name or name and address of the manufacturer or importer/distributor ※The items marked with * are not necessarily found in all cosmetics.
Other notes
(1) Matters requiring labeling on external containers, etc. (Article 51 of the Act applied mutatis mutandis to Article 62 of the Act)
When matters that must be indicated on the immediate container (encapsulation) cannot be easily seen through the outer container (envelope), the same information must be indicated on the outer container (envelope). must be listed.
(2) Location and terms of labeling (Article 53 of the Act applied mutatis mutandis to Article 62 of the Act)
Display items must be displayed in a place that is easier to see than other characters, articles, drawings or designs, and must be accurately described in terms that are easy for general purchasers and users to read and understand.
(3) Obligation to clearly state (Article 217 of the Ordinance for Enforcement applied mutatis mutandis to Article 228 of the Ordinance for Enforcement)
The labeling on the attached document or on its container (encapsulation) must be particularly clear.
(4) Description in Japanese (Article 218 of the Ordinance for Enforcement applied mutatis mutandis to Article 228 of the Ordinance for Enforcement)
Matters stipulated in Articles 51 and 52, which are applied mutatis mutandis in Articles 61 and 62 of the Law (immediate container, external container, matters to be described in package inserts) must be written in Japanese.
Penalty provisions
If you sell cosmetics that do not contain the items stipulated in the Pharmaceuticals and Medical Devices Act, you may be sentenced to imprisonment for not more than two years, a fine of not more than 2 million yen , or both. (Law, Article 85, Item 3)
I can't find the information I'm looking for
Creator of this page and contact information
Pharmaceutical Guidance Group, Sanitation Section, Health Promotion Department, Health Promotion Department, Osaka City Government
Address: 1-3-20 Nakanoshima, Kita-ku, Osaka 530-8201 (Osaka City Hall 2F)
Phone: 06-6208-9986
Fax: 06-6202-6967
